Fetuins and related proteins
Fetuin-A/alpha-2-Heremans Schmid's glycoprotein (FETUA/AHSG), Fetuin-B (FETUB), Histidine-rich glycoprotein (HRG) and Kininogen (KNG) are members of the cystatin superfamily, a subgroup of cysteine proteinase inhibitors. They form the so-called type 3 members, which are all synthesized in the liver and secreted into the blood. All type 3 cystatins have several disulfide bridges. Each protein is encoded by a single gene. The genes are located in close proximity in all known vertebrate genomes and are closely related. Figure 1 schematically shows the protein structure of type 3 cystatins.
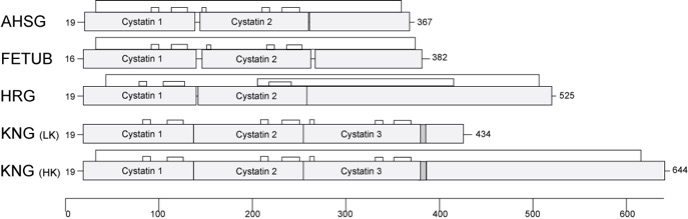
Figure 1 Structural comparison of type 3 cystatins. Fetuin-A (AHSG), fetuin-B (FETUB), histidine-rich glycoprotein (HRG) and kininogen (KNG), subdivided in low molecular weight kininogen (LK) and high molecular weight kininogen (HK), are type 3 members of the cystatin superfamily. Characteristically they present at least two aminoterminal cystatin domains with disulfide bridges in distinct distances indicated by the black lines above each domain structure. Numbers at the left represent the length of the leader sequence, numbers at the right the amino acid length in total.
The fetuin genes were created by duplication of cystatin protein domains and fusion with other stable gene units. The combination of stable protein building blocks conserved during evolution produced proteins that are very similar in structure but have completely different functions. This can be best explained using the example of the proteins Fetuin-A and Fetuin-B. Both proteins have three domains, are similar in size, are made mainly in the liver and are secreted into the blood. Through genetic studies on mouse strains from which the respective genes were specifically removed, using so-called knockout mice, we found out that Fetuin-A is mainly responsible for the transport and stability of minerals in the body, whereas Fetuin-B is highly specific in inhibiting so-called zinc metalloproteinases. Despite the close relationship of the proteins, the knockout mice therefore show strikingly different phenotypes, i.e. the lack of Fetuin-A and Fetuin-B has completely different consequences. Fetuin-A knockout mice show calcium deposits throughout the body, which lead to restrictions in organ function with increasing age, whereas Fetuin-B knockout mice are completely infertile as females, because the shell of the eggs of these females harden before fertilisation and thus prevent fertilisation.
In addition to these clearly detectable functions, both proteins probably have other, subordinate functions that are emphasised elsewhere but which cannot be detected in the knockout mice. This does not mean that such functions cannot be observed experimentally, but in contrast to the functions just described, they cannot be consistently demonstrated by genetic, biochemical, molecular biological and clinical studies, but often only by individual observation on selected experimental systems.
Introductions to the function and mode of action of the fetuin proteins can be found in the following review articles and the literature cited therein, as well as in the list of literature which opens by clicking on the menu item "Publications". For further details see "Biomineralisation" and "Reproductive Biology" on the homepage. Enjoy reading!
Further Reading
Type 3 cystatins; fetuins, kininogen and histidine-rich glycoprotein.
Lee C, Bongcam-Rudloff E, Sollner C, Jahnen-Dechent W, Claesson-Welsh L.
Front Biosci (Landmark Ed). 2009 Jan 1;14:2911-22. Review.
PMID: 19273244
Nature's remedy to phosphate woes: calciprotein particles regulate systemic mineral metabolism.
Jahnen-Dechent W, Smith ER.
Kidney Int. 2020 Apr;97(4):648-651. doi: 10.1016/j.kint.2019.12.018. PMID: 32200857
Fetuin-A regulation of calcified matrix metabolism.
Jahnen-Dechent W, Heiss A, Schäfer C, Ketteler M.
Circ Res. 2011 Jun 10;108(12):1494-509. doi: 10.1161/CIRCRESAHA.110.234260. Review. PMID: 21659653
Home / Publications / Teaching / Team / Contact




